181 | Add to Reading ListSource URL: research.wayne.eduLanguage: English - Date: 2016-06-21 15:22:10
|
|---|
182 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
183 | Add to Reading ListSource URL: fsns.comLanguage: English - Date: 2016-04-08 12:51:19
|
|---|
184 | Add to Reading ListSource URL: www.amp.orgLanguage: English - Date: 2015-12-17 10:50:39
|
|---|
185![34268 § 71.1 Federal Register / Vol. 81, NoTuesday, May 31, Rules and Regulations [Amended] 34268 § 71.1 Federal Register / Vol. 81, NoTuesday, May 31, Rules and Regulations [Amended]](https://www.pdfsearch.io/img/ff32d58caff42590807bf94f3d3a6cc5.jpg) | Add to Reading ListSource URL: cordellbank.noaa.govLanguage: English - Date: 2016-06-01 12:38:52
|
|---|
186 | Add to Reading ListSource URL: humansubjects.stanford.eduLanguage: English - Date: 2016-04-12 01:21:50
|
|---|
187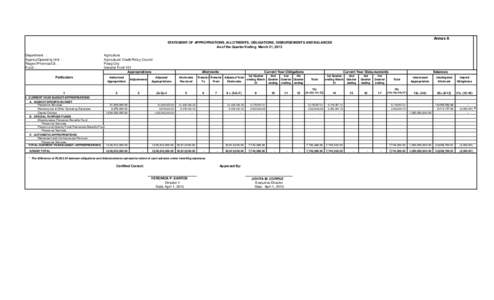 | Add to Reading ListSource URL: acpc.gov.phLanguage: English - Date: 2015-06-17 22:59:05
|
|---|
188 | Add to Reading ListSource URL: www.luitpoldanimalhealth.comLanguage: English - Date: 2016-04-13 11:34:05
|
|---|
189 | Add to Reading ListSource URL: www.afdo.orgLanguage: English - Date: 2015-07-08 09:57:27
|
|---|
190 | Add to Reading ListSource URL: www.mvpharm.comLanguage: English - Date: 2016-07-11 16:59:56
|
|---|